en
names in breadcrumbs


Nematodes within the Secernentea have phasmids, which are unicellular glands. Phasmids likely function as chemoreceptors. Females may produce pheromones to attract males.
Nematodes in general have papillae, setae and amphids as the main sense organs. Setae detect motion (mechanoreceptors), while amphids detect chemicals (chemoreceptors).
Communication Channels: tactile ; chemical
Other Communication Modes: pheromones
Perception Channels: tactile ; chemical
The mosquito ingests Dirofilaria immitis larvae, microfilariae (first stage juvenile, L1), after a blood meal from a dog or other infected host. The first stage larvae (L1) develop into second stage larvae (L2) in the malpighian tubes. Then the third stage larvae (L3) develop and enter the body cavity, the hemoceol. Development from the microfilariae to the third larval stage takes 15-16 days. The L3 escape in a pool of hemolymph as the mosquito inserts its labium into the definitive host, which is usually a dog. If its stylets are withdrawn, the L3 enter through the puncture wound made by the mosquito. The L3 will then molt into the fourth larval stage (L4) in the definitive host 0-14 days after infection. The L4 migrate to submuscular membranes and subcutaneous tissue and remain dormant. They molt into the fifth larval stage (L5) and migrate through venule walls and end up in the pulmonary arterioles and right heart of the definitive host. The L5 will mature into adults that will further migrate to the right ventricle or pulmonary artery 85-120 days after infection. The adult will reach maturity in a further 2-month period and shed microfilariae in the blood to begin the cycle all over again.
The adults can remain in the heart or artery for up to 7 years. Microfilariae can remain in the circulation of the mosquito for up to 2 years. In humans the parasites can not reach the adult stages and remain in the larval stages. No microfilariae are ever present in the blood of humans because the parasites can never fully develop to shed the microfilariae into the blood.
Dirofilaria immitis is of veterinary importance because it threatens the health of dogs, cats and other animals, including humans. The dog heartworm causes pulmonary dirofilariosis in humans when immature worms accidentally infect humans and begin developing in nodules in the lungs or other subcutaneous tissue. In heavy infections of dogs, cats and other wildlife, the worms can cause circulatory distress, interference with functions of the heart valves and cause congestion of the right side of the heart. Cirrhosis of the liver and endarteritis can become apparent after 9-10 months if no treatment is administered. Initially in dogs the clinical signs include decreased exercise endurance, chronic cough and collapse after excercise. In humans the clinical symptoms are less apparent including lack of stamina. Diagnosis in animals includes testing the blood for microfilariae and performing a thoracic radiography. Administering a lung biopsy and chest x-ray is the primary diagnostic tool in humans. In order to temporarily control this disease in humans it is important keep dogs indoors during peak mosquito biting hours, which is at night. This will control the infection of the reservoir host. Also, spraying to abate mosquitoes would control the disease by decreasing the vector population. The adaptability of some vector mosquito species particularly in the genus Culex, plays an important role in the spread of infection. To control the transmission of D. immitis in domestic animals, preventative techniques administered by regular veterinary visits can drastically reduce the risk of infection. For example, Selamectin is a liquid applied topically on a dog or cat that safely prevents the transmission of the adult heartworm. Dogs living in open air and poor conditions seem to be more prone to becoming infected.
Negative Impacts: injures humans (causes disease in humans ); causes or carries domestic animal disease
The mosquito ingests Dirofilaria immitis larvae, microfilariae (first stage juvenile, L1), after a blood meal from a or other infected host. Larvae are then passed back to dogs where they develop into adults.
Ecosystem Impact: parasite
Species Used as Host:
The developing Dirofilaria immitis larvae feed on the cells of the malpighian tubes in the intermediate host, the mosquito. The adult D. immitis feed on blood and nutrients in the circulation of the dog or other host. Pharyngeal glands and intestinal epithelium produce digestive enzymes to feed on the hosts’ body fluids. Extracellular digestion begins within the lumen and is finished intracellularly.
Animal Foods: body fluids
Primary Diet: carnivore (Eats body fluids)
Dirofilaria immitis is found in Southern Europe, India, China, Japan, Australia and North and South America.
Biogeographic Regions: nearctic ; palearctic ; oriental ; neotropical ; australian
Dirofilaria immitis is found in many tropical, subtropical and temperate regions of the world, particularly humid areas and river valleys where environmental conditions harbor the breeding of mosquito vectors.
The intermediate host may belong to several species of mosquitos such as the Aedes, Anopholes, and Culex. The primary definitive host of D. immitis is the dog. However, other abnormal definitive hosts are cats (produces a different syndrome than in the dog), fox, coyote, wolf, sea lions, harbor seals, laboratory ferrets, horses, bears, raccoons, wolverines, muskrats and red pandas. Larvae do not grow to adults in humans.
Habitat Regions: temperate ; tropical ; terrestrial
Terrestrial Biomes: desert or dune ; savanna or grassland ; chaparral ; forest ; rainforest ; scrub forest ; mountains
Aquatic Biomes: lakes and ponds; rivers and streams; temporary pools; coastal
Wetlands: marsh ; swamp ; bog
Other Habitat Features: urban ; suburban ; agricultural ; riparian
Dirofilaria immitis is a cylindrical, slender white worm. As a nematode, it has a cuticle with three main outer layers made of collagen and other compounds. The outer layers are non-cellular and are secreted by the epidermis. The cuticle layer protects the nematodes so they can invade the digestive tracts of animals.
Nematodes have longitudinal muscles along the body wall. The muscles are obliquely arranged in bands. Dorsal, ventral and longitudinal nerve cords are connected to the main body of the muscle.
Both sexes are different. The adult male, measuring 12-16 cm, is smaller than the adult female, which is 25-30 cm. The male has a posterior end spirally coiled and a tail with many alae, which are thickenings of the cuticle. The female posterior is straight. Both sexes have a mouth, a filariform esophogus, anal pore, excretory pore and a nerve ring. The male has a seminal vesicle and testis while the female bears an ovary and oviduct.
The larvae, called microfilariae, are 307-322 micrometers long and 6.7-7.1 micrometers wide. They have a straight posterior end regardless of the sex and a tapered anterior end. They have no cephalic hook and are not ensheathed.
Range length: 12 to 30 cm.
Other Physical Features: ectothermic ; heterothermic ; bilateral symmetry
Sexual Dimorphism: female larger; sexes shaped differently
As parasites, these animals are not usually preyed on directly, but are ingested from host to host.
Females may produce a phermomone to attract males. The male coils around a female with his curved area over the female genital pore. The gubernaculum, made of cuticle tissue, guides spicules which extend through the cloaca and anus. Males use spicules to hold the female during copulation. Nematode sperm are amoeboid-like and lack flagella.
Key Reproductive Features: sexual ; fertilization (Internal ); ovoviviparous
Parental Investment: pre-fertilization (Provisioning)
Dirofilaria immitis, also known as heartworm or dog heartworm, is a parasitic roundworm that is a type of filarial worm, a small thread-like worm, that causes dirofilariasis. It is spread from host to host through the bites of mosquitoes. There are four genera of mosquitoes that transmit dirofilariasis, Aedes, Culex, Anopheles, and Mansonia.[1] The definitive host is the dog, but it can also infect cats, wolves, coyotes, jackals, foxes, ferrets, bears, seals, sea lions and, under rare circumstances, humans.[2]
Adult heartworms often reside in the pulmonary arterial system (lung arteries) as well as the heart, and a major health effect in the infected animal host is a manifestation of damage to its lung vessels and tissues.[3] In cases involving advanced worm infestation, adult heartworms may migrate to the right heart and the pulmonary artery. Heartworm infection may result in serious complications for the infected host if left untreated, eventually leading to death, most often as a result of secondary congestive heart failure.
Although at one time confined to the southern United States, heartworm has now spread to nearly all locations where its mosquito vector is found. In the southeast region of the United States, veterinary clinics saw an average of more than 100 cases of heartworm each in 2016.[4] Transmission of the parasite occurs in all of the United States (cases have even been reported in Alaska), and the warmer regions of Canada. The highest infection rates are found within 150 miles of the coast from Texas to New Jersey, and along the Mississippi River and its major tributaries.[3] It has also been found in South America,[5] southern Europe,[6][7] Southeast Asia,[8] the Middle East,[9] Australia, Korea, and Japan.[10][11]

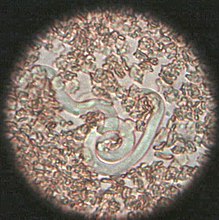
Heartworms go through several life stages before they become adults infecting the pulmonary artery of the host animal. The worms require the mosquito as an intermediate host to complete their lifecycles. The rate of development in the mosquito is temperature-dependent, requiring about two weeks of temperature at or above 27 °C (80 °F). Below a threshold temperature of 14 °C (57 °F), development cannot occur, and the cycle is halted.[12] As a result, transmission is limited to warm weather, and duration of the transmission season varies geographically. The period between the initial infection when the dog is bitten by a mosquito and the maturation of the worms into adults living in the pulmonary arteries takes six to seven months in dogs and is known as the "prepatent period".
The first larval stage (L1) and second larval stage (L2) of heartworm development occurs within the body of a mosquito. Once the larvae develop into the infective third larval stage (L3), the mosquito locates and bites a host, depositing the larvae under the skin at the site of the bite. After a week or two of further growth, they molt into the fourth larval stage (L4) . Then, they migrate to the muscles of the chest and abdomen, and 45 to 60 days after infection, molt to the fifth stage (L5, immature adult). Between 75 and 120 days after infection, these immature heartworms then enter the bloodstream and are carried through the heart to reside in the pulmonary artery. Over the next three to four months, they increase greatly in size. The female adult worm is about 30 cm in length, and the male is about 23 cm, with a coiled tail.[13] By seven months after infection, the adult worms have mated and the females begin giving birth to live young, called microfilariae. Heartworms can live for 5 to 7 years in a dog.[14]
The microfilariae circulate in the bloodstream for as long as two years, and are ingested by bloodsucking mosquitos, where development occurs and the cycle repeats.
Hosts of Dirofilaria immitis include:[2]
Reservoir hosts for D. immitis are coyotes and stray dogs.[17]
Dogs show no indication of heartworm infection during the six-month prepatent period prior to the worms' maturation, and current diagnostic tests for the presence of microfilariae or antigens cannot detect prepatent infections. Rarely, migrating heartworm larvae get "lost" and end up in aberrant sites, such as the eye, brain, or an artery in the leg, which results in unusual symptoms such as blindness, seizures, and lameness, but normally, until the larvae mature and congregate inside the heart, they produce no symptoms or signs of illness.
Many dogs show little or no sign of infection even after the worms become adults. These animals usually have only a light infection and live a fairly sedentary lifestyle. However, active dogs and those with heavier infections may show the classic signs of heartworm disease. Early signs include a cough, especially during or after exercise, and exercise intolerance. In the most advanced cases where many adult worms have built up in the heart without treatment, signs progress to severe weight loss, fainting, coughing up blood, and finally, congestive heart failure.
There are 4 different classes of symptoms:
Wolbachia pipientis is an intracellular bacterium that is an endosymbiont of D. immitis. All heartworms are thought to be infected with Wolbachia to some degree. The inflammation occurring at the die-off of adult heartworms or larvae is in part due to the release of Wolbachia bacteria or protein into the tissues. This may be particularly significant in cats, in which the disease seems to be more related to larval death than living adult heartworms. Treating heartworm-positive animals with an antibiotic such as doxycycline to remove Wolbachia may prove to be beneficial, but further studies are necessary.[19]
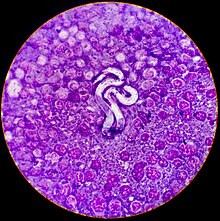
Microfilarial detection is accomplished by the using one of the following methods:
Direct blood smear
A blood sample is collected and viewed under the microscope. The direct smear technique allows examination of larval motion, confirming the presence of microfilaria. It also helps in the distinction of D. immitis from Acanthocheilonema reconditum. This distinction is important because the presence of the latter parasite does not pose a health risk to the host. D. immitis usually has stationary body movement, while A. reconditum has progressive movement. However, this method often misses light infections because only a small amount of blood sample is used.[21]
Hematocrit tube method
This method uses a microhematocrit (or capillary tube) filled with a blood sample that has been centrifuged, separating the plasma from the red blood cells. These layers are divided by the buffy coat. The buffy coat consists of the leukocytes and platelets that are in the sample. The tube is snapped at the buffy coat and added to a slide for microscopic examination. Adding methylene blue stain to the sample may allow greater visibility of any microfilariae. However, the hematocrit tube method will not allow for species differentiation.[22]
Modified Knott's test
The modified Knott's test is more sensitive because it concentrates microfilariae, improving the chance of diagnosis.[3] A blood sample is mixed with 2% formalin and centrifuged in a tube. The supernatant is removed and methylene blue stain is added to the pellet remaining in the tube for microscopic examination. It allows microfilariae species differentiation based on morphology. Microfilariae can be differentiated between D. immitis and Acanthocheilonema reconditum because of small differences in morphology. The Modified Knott's test is the best method of visual examination when determining presence of microfilaria because it preserves their morphology and size. It is easy to perform, quick, and inexpensive.[23]
The potential for a microfilaremic infection is 5 – 67%. The number of circulating microfilariae does not correlate with the number of adult heartworms, so is not an indicator of disease severity.[3]
Antigen testing
In most practices, antigen testing has supplanted or supplemented microfilarial detection. Combining the microfilaria and adult antigen test is most useful in dogs receiving diethylcarbamazine or no preventive (macrolides like ivermectin or moxidectin typically render the dog amicrofilaremic). Up to 1% of infected dogs are microfilaria-positive and antigen-negative.[3] Immunodiagnostics (ELISA, lateral flow immunoassay, rapid immunomigration techniques) to detect heartworm antigen in the host's blood are now regularly used. They can detect occult infections, or infections without the presence of circulating microfilariae. However, these tests are limited in that they only detect the antigens released from the sexually mature female worm's reproductive tract. Therefore, false-negative results may occur during the first five to eight months of infection when the worms are not yet sexually mature.[3] The specificity of these tests is close to 100%, and the sensitivity is more than 80%.[24] A recent study demonstrated a sensitivity of only 64% for infections of only one female worm, but improved with increasing female worm burden (85%, 88%, and 89% for two, three, and four female worms, respectively). Specificity in this study was 97%.[3] False-negative test results can be due to low worm counts, immature infections, and all-male infections.
X-rays
X-rays are used to evaluate the severity of the heartworm infection and develop a prognosis for the animal. Typically, the changes observed are enlargement of the main pulmonary artery, the right side of the heart, and the pulmonary arteries in the lobes of the lung. Inflammation of the lung tissue is also often observed.[25]
If an animal is diagnosed with heartworms, treatment may be indicated. Before the worms can be treated, however, the dog's heart, liver, and kidney function must be evaluated to determine the risks of treatment. Usually, the adult worms are killed with an arsenic-based compound. The currently approved drug in the US, melarsomine, is marketed under the brand name Immiticide.[26] It has a greater efficacy and fewer side effects than the previously used drug thiacetarsamide, sold as Caparsolate, which makes it a safer alternative for dogs with late-stage infections.
After treatment, the dog must rest, and exercise is to be heavily reduced for several weeks so as to give its body sufficient time to absorb the dead worms without ill effect. Otherwise, if the dog is under exertion, dead worms may break loose and travel to the lungs, potentially causing respiratory failure and sudden death. According to the American Heartworm Society, the administering of aspirin to dogs infected with heartworms is no longer recommended due to a lack of evidence of clinical benefit, and aspirin may be contraindicated in several cases. Aspirin had previously been recommended for its effects on platelet adhesion and the reduction of vascular damage caused by the heartworms.
The course of treatment is not completed until several weeks later, when the microfilariae are dealt with in a separate course of treatment. Once heartworm tests are negative and no surviving worm is detected, the treatment is considered a success, and the patient is effectively cured.
Surgical removal of the adult heartworms as a form of treatment may also be indicated, especially in advanced cases with substantial heart involvement and damage.[27]
Prevention of heartworm infection can be obtained through a number of veterinary drugs. The drugs approved for use in the US are ivermectin (sold under the brand names Heartgard, Iverhart, and several other generic versions), milbemycin (Interceptor Flavor Tabs and Sentinel Flavor Tabs) and moxidectin (Simparica Trio) administered as chewable tablets. Moxidectin is also available in both a six-month and 12-month sustained-release injection, ProHeart 6 and ProHeart 12, respectively, administered by veterinarians. This injectable form of moxidectin was taken off the market in the United States due to safety concerns in 2004, but the FDA returned a newly formulated ProHeart 6 to the market in 2008. ProHeart 6 remains on the market in many other countries, including Canada and Japan. Its sister product, ProHeart 12, is used extensively in Australia and Asia as a 12-month injectable preventive. It was approved for use in the United States by the FDA in July 2019.[28] Topical treatments are available, as well. Advantage Multi (imidacloprid plus moxidectin) Topical Solution, uses moxidectin for control and prevention of roundworms, hookworms, heartworms, and |whipworms, as well as imidacloprid to kill adult fleas. Selamectin (Revolution) is a topical preventive likewise administered monthly, and can also be used to control fleas, ticks, and mites.
Preventive drugs are highly effective, and when regularly administered, have been shown to protect more than 99% of dogs and cats from heartworm. Most compromises in protection result from the failure to properly administer the drugs during seasonal transmission periods.[29] In regions where the temperature is consistently above 14 °C (57 °F) year-round, a continuous prevention schedule is recommended.
Due to newly emerging resistant strains of heartworms, which no macrocyclic lactone (heartworm prevention) can protect against, the American Heartworm Society recommends dogs be on a repellent and a heartworm preventive. The repellent, such as Vectra 3-D, keeps mosquitoes from feeding on the dog and transmitting the L3 stage worms. If a dog is bitten, the heartworm preventive takes over when administered. If a mosquito feeds on a heartworm positive dog on a repellent, they do not live long enough for the microfilaria they ingested to molt into the infective L3 larva. Vectra 3-D was tested using thousands of mosquitoes infected with the resistant heartworm strain JYD34. In the control group that was given only a placebo, every dog contracted heartworms. In the experimental group that was given only Vectra 3-D, two of eight dogs contracted heartworms and had an average of 1.5 adult worms each. In the experimental group given both heartworm prevention and Vectra 3-D, one dog was infected with L3 stage larvae that did not mature into adulthood due to the heartworm prevention. Using a repellent and a prevention is at least 95% effective.[30][31]
Ivermectin, even with lapses up to four months between doses, still provides 95% protection from adult worms. This period is called the reach-back effect.[32] Since dogs are susceptible to heartworms, they should be tested annually before they start preventive treatment.[17] Annual heartworm testing is highly recommended for pet owners who choose to use minimal dosing schedules. Testing a dog annually for heartworms and other internal parasites is a fundamental part of a complete heartworm prevention program, and is also recommended for dogs who are already on a monthly prevention program.[17]
While dogs are a natural host for D. immitis, cats are atypical hosts. Because of this, differences between canine and feline heartworm diseases are significant. The majority of heartworm larvae do not survive in cats, so unlike in dogs, a typical infection in cats is two to five worms. The lifespan of heartworms is considerably shorter in cats, only two to three years, and most infections in cats do not have circulating microfilariae. Cats are also more likely to have aberrant migration of heartworm larvae, resulting in infections in the brain or body cavities.[33]
The infection rate in cats is 1–5% of that in dogs in endemic areas.[34] Both indoor and outdoor cats are infected. The mosquito vector is known to enter homes.[35]
The vascular disease in cats that occurs when the L5 larvae invade the pulmonary arteries is more severe than in dogs. A reaction has been identified in cats: heartworm-associated respiratory disease, which can occur three to four months after the initial infection, and is caused by the presence of the L5 larvae in the vessels. The subsequent inflammation of the pulmonary vasculature and lungs can be easily misdiagnosed as feline asthma or allergic bronchitis.[36]
Obstruction of pulmonary arteries due to emboli from dying worms is more likely to be fatal in cats than dogs because of less collateral circulation and fewer vessels.[37] Heartworms can live for 2 to 3 years in cats.[14]
Acute heartworm disease in cats can result in shock, vomiting, diarrhea, fainting, and sudden death. Chronic infection can cause loss of appetite, weight loss, lethargy, exercise intolerance, coughing, and difficulty breathing. Some cats' immune systems are able to clear a heartworm infection, though the immune system response can cause many of the same symptoms. Also, even if the infection resolves, respiratory damage can cause some symptoms to persist beyond it.[36][38]
Diagnosis of heartworm infection in cats is problematic. Like in dogs, a positive ELISA test for heartworm antigen is a very strong indication of infection. However, the likelihood of a positive antigen test depends on the number of adult female worms present. If only male worms are present, the test will be negative. Even with female worms, an antigen test usually only becomes positive seven to eight months after infection. Therefore, a cat may have significant clinical signs long before the development of a positive test. Heartworm-associated respiratory disease can be found in cats that never develop adult heartworms and therefore never have a positive antigen test.
An antibody test is also available for feline heartworm infection. It will be positive in the event of exposure to D. immitis, so a cat that has successfully eliminated an infection may still be positive for up to three months. The antibody test is more sensitive than the antigen test, but it does not provide direct evidence of adult infection.[39] It can, however, be considered specific for diagnosing previous larval infections, and therefore fairly specific for heartworm-associated respiratory disease.
X-rays of the chest of a heartworm-infected cat may show an increased width of the pulmonary arteries and focal or diffuse opacities in the lungs. Echocardiography is a fairly sensitive test in cats. Adult heartworms appear as double-lined hyperechoic structures within the heart or pulmonary arteries.[40]

Heartworm prevention for cats is available as ivermectin (Heartgard for Cats), milbemycin (Interceptor), or the topical selamectin (Revolution for Cats) and Advantage Multi (imidacloprid + moxidectin) topical solution. Ivermectin, milbemycin, and selamectin are approved for use in cats in the US.
Arsenic compounds have been used for heartworm adulticide treatment in cats, as well as dogs, but seem more likely to cause pulmonary reactions. A significant number of cats develop pulmonary embolisms a few days after treatment. The effects of melarsomine are poorly studied in cats. Due to a lack of studies showing a clear benefit of treatment and the short lifespan of heartworms in cats, adulticide therapy is not recommended, and no drugs are approved in the US for this purpose in cats.[37]
Treatment typically consists of putting the cat on a monthly heartworm preventive and a short-term corticosteroid.[33] Surgery has also been used successfully to remove adult worms. The prognosis for feline heartworm disease is guarded.
Dirofilaria are important medical parasites, but diagnosis is unusual and is often only made after an infected person happens to have a chest X-ray following granuloma formation in the lung. The nodule itself may be large enough to resemble lung cancer on the X-ray, and requires a biopsy for a pathologic assessment. This has been shown to be the most significant medical consequence of human infection by the canine heartworm. Patients are infected with the parasite through the bite of an infected mosquito, which is the same mechanism that causes heartworm infection in dogs.[41]
D. immitis is one of many species that can cause infection in dogs and humans. It was thought to infect the human eye, with most cases reported from the southeastern United States. However, these cases are now thought to be caused by a closely related parasite of raccoons, Dirofilaria tenuis. Several hundred cases of subcutaneous infections in humans have been reported in Europe, but these are almost always caused by another closely related parasite, Dirofilaria repens, rather than the dog heartworm. There are proven D. immitis infections,[16] but humans rarely get infected with heartworms due to the larvae never fully maturing.[41] When the heartworm microfilariae migrate through the skin, they often die since heartworms cannot survive in a human host, even if they make it into the bloodstream.[41] Once the heartworms die, the immune system in the human body reacts to their tissue with inflammation as it tries to destroy the heartworms.[41] When this happens, the condition is called pulmonary dirofilariasis.[41] Heartworms in humans is not a serious problem unless they are causing pain, discomfort, and other noticeable symptoms.[41]
Dirofilaria immitis, also known as heartworm or dog heartworm, is a parasitic roundworm that is a type of filarial worm, a small thread-like worm, that causes dirofilariasis. It is spread from host to host through the bites of mosquitoes. There are four genera of mosquitoes that transmit dirofilariasis, Aedes, Culex, Anopheles, and Mansonia. The definitive host is the dog, but it can also infect cats, wolves, coyotes, jackals, foxes, ferrets, bears, seals, sea lions and, under rare circumstances, humans.
Adult heartworms often reside in the pulmonary arterial system (lung arteries) as well as the heart, and a major health effect in the infected animal host is a manifestation of damage to its lung vessels and tissues. In cases involving advanced worm infestation, adult heartworms may migrate to the right heart and the pulmonary artery. Heartworm infection may result in serious complications for the infected host if left untreated, eventually leading to death, most often as a result of secondary congestive heart failure.